On Friday, April 12, 2024; the U.S. Food and Drug Administration (FDA) hosted an Oncologic Drugs Advisory Committee (ODAC) meeting to examine the use of minimal residual disease (MRD) as a clinical trial endpoint in multiple myeloma, specifically discussing assessment timing, relevant patient groups, and trial designs aimed at supporting accelerated approval of new therapies.
The FDA defines an advisory committee as a group that offers independent scientific expertise on general topics or specific products to guide FDA decisions. While advisory committee recommendations are not legally binding, the FDA generally considers them carefully.
The International Myeloma Foundation (IMF), alongside the collaborative research group i2TEAMM (International Independent Team for Endpoint Approval of Myeloma MRD), participated prominently, joined by representatives from the Sylvester Comprehensive Cancer Center at the University of Miami, led by Dr. Carl Ola Landgren. Key members of i2TEAMM in attendance included myself as the IMF’s Chief Scientific Officer; Bruno Paiva, PhD (University of Navarra, Spain); Qian Shi, PhD (Mayo Clinic, MN); and Kenneth C. Anderson, MD (Dana-Farber Cancer Institute, MA).
i2TEAMM’s objective in the meeting was to validate MRD as a surrogate endpoint of progression-free survival (PFS) in multiple myeloma trials, based on extensive patient-level data across randomized trials. Despite challenges, i2TEAMM’s analysis highlighted that MRD negativity at nine and twelve months is a strong predictor of clinical benefit, consistently correlating with improved PFS and overall survival across multiple patient settings.
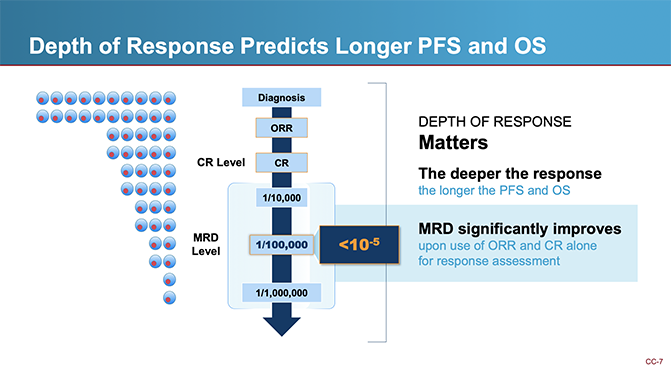
During the presentations, I introduced the importance of deep response, emphasizing how MRD provides a far more sensitive measure of treatment success than traditional response rates. Dr. Paiva discussed MRD testing advancements, Dr. Shi presented key meta-analysis findings, and Dr. Anderson summarized the results. These presentations reinforced MRD testing’s prognostic power and its potential as an endpoint to guide accelerated approval in myeloma clinical trials.
At the close of deliberations, ODAC members unanimously endorsed MRD testing as an early approval endpoint (12-0), marking a historic shift. If FDA-approved, MRD testing could enable faster access to novel therapies for myeloma patients. The IMF will continue to provide updates on the FDA’s final decision.



